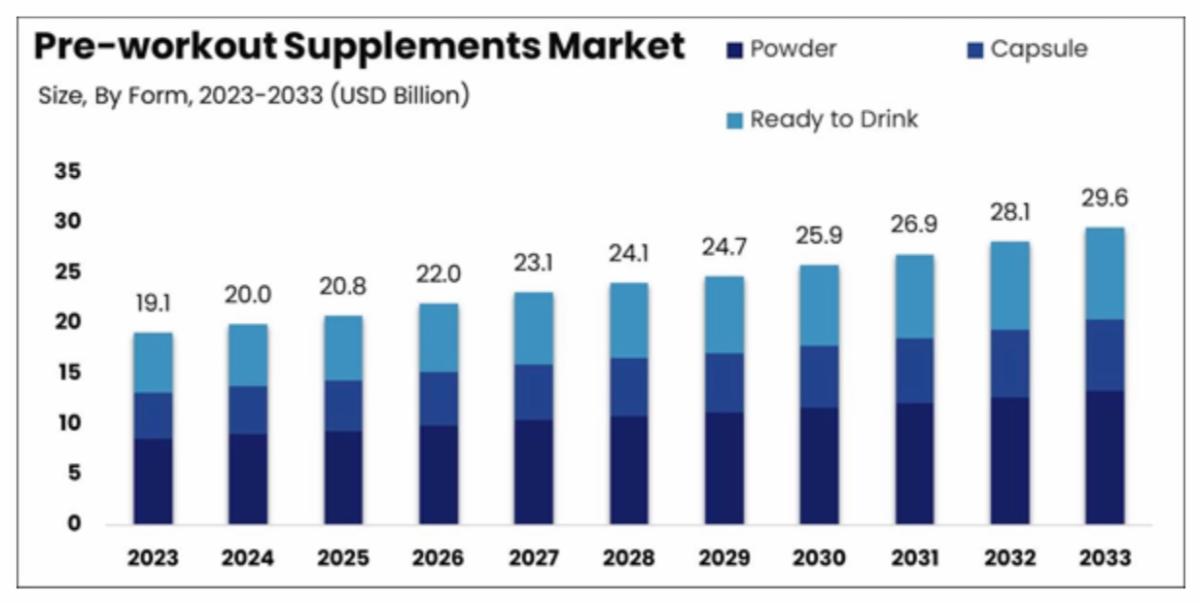
| Aspire Biopharma (NASDAQ: ASBP) is making a move into the high-growth pre-workout supplement industry, which is projected to see significant expansion, reaching $29B by 2033.
The company recently announced plans to launch a single-dose pre-workout supplement in 2025, utilizing its patent-pending and proprietary sublingual delivery technology.
Unlike traditional pre-workout supplements that require mixing with water and waiting for effects, Aspire Biopharma (NASDAQ: ASBP)’s fast-absorbing formulation is designed to work within minutes, offering a major advantage in the performance supplement market.
By combining science-backed performance enhancement with a next-generation delivery method, Aspire Biopharma (NASDAQ: ASBP) is positioning itself as a key innovator in this expanding market.
7 Reasons Why Aspire Biopharma (NASDAQ: ASBP) is Topping Our Watchlist Tomorrow Morning…
1. Massive Market Potential: A patented sublingual absorption system is targeting a $100B market, offering a faster, more efficient, and potentially safer way to deliver widely used treatments—placing Aspire Biopharma (NASDAQ: ASBP) at the forefront of this innovation.
2. Recent Nasdaq Listing: As a new and relatively unknown name on the Nasdaq as of February 20, 2025, Aspire Biopharma (NASDAQ: ASBP) is still flying under the radar—but that may not last for long. With its cutting-edge technology and expanding pipeline, this listing could be the catalyst that brings increased visibility and traction to the company.
3. Breakthrough in Emergency Treatment: A sublingual aspirin formulation designed to deliver therapeutic levels in under two minutes could be a game-changer for cardiovascular emergencies, and Aspire Biopharma (NASDAQ: ASBP) is actively advancing its development.
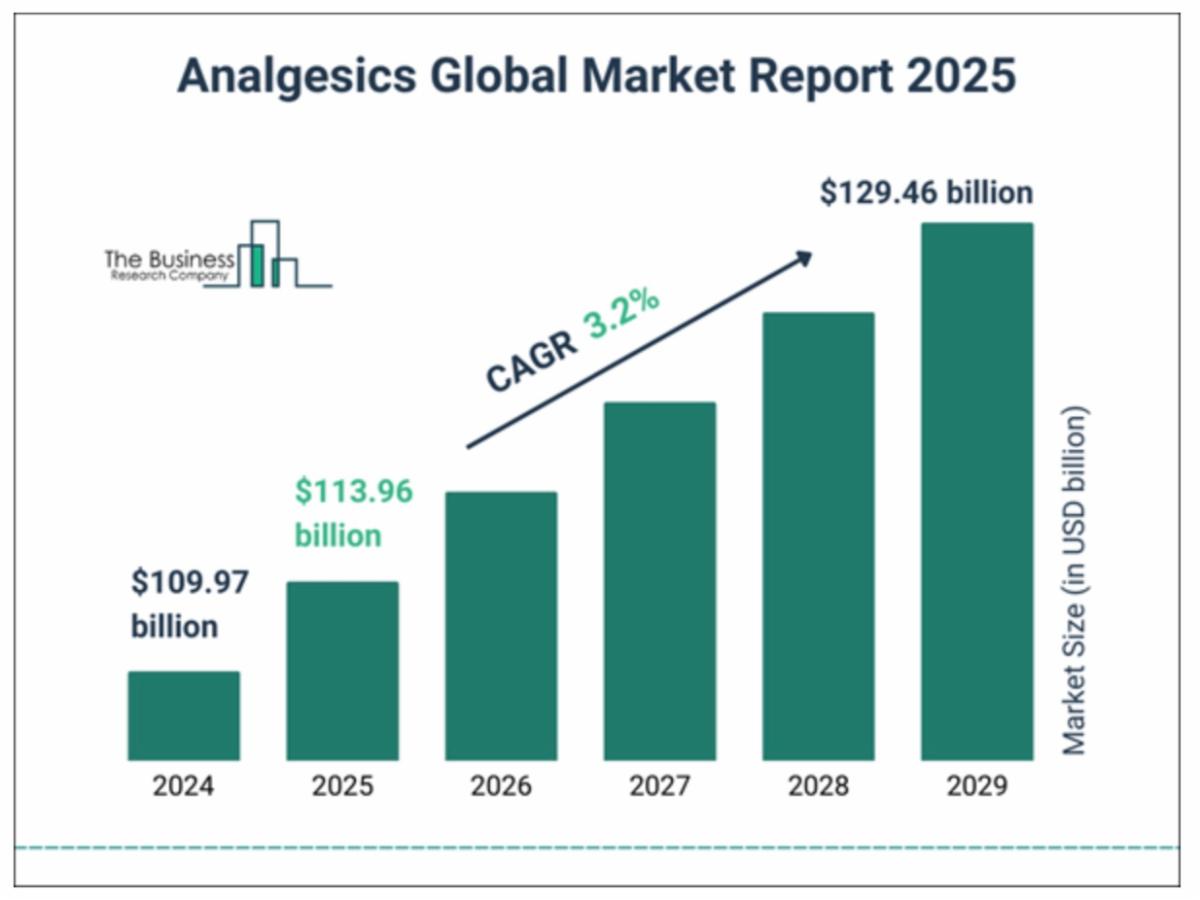
4. Expanding Into the $100B Analgesics Market: The global pain relief industry continues to grow, and Aspire Biopharma (NASDAQ: ASBP) is introducing a faster-acting, sublingual aspirin that bypasses the digestive tract—potentially offering a more efficient pain management solution.
5. Strategic Industry Partnerships: Contract manufacturers are being engaged for production, with Glatt Air Techniques already supporting clinical trials and ThermoFisher Scientific in discussions for additional manufacturing, as Aspire Biopharma (NASDAQ: ASBP) aims to scale its sublingual delivery system.
6. Upcoming Clinical Milestones: A Phase 1 clinical trial for sublingual aspirin is on track for April 2025, marking a significant step toward regulatory approval, with Aspire Biopharma (NASDAQ: ASBP) progressing through the testing process.
7. Next-Gen Absorption Technology: A sublingual pre-workout supplement is set to launch in 2025, eliminating the need for mixing powders while allowing for rapid absorption, as Aspire Biopharma (NASDAQ: ASBP) works with Desert Stream Inc. to bring this product to market.
Final Thoughts on Aspire Biopharma (NASDAQ: ASBP)
Let’s be real—the biotech and pharmaceutical space moves fast, and the ones who spot breakthroughs early often have the upper hand.
Aspire Biopharma (NASDAQ: ASBP) has put itself in the mix with a first-of-its-kind sublingual absorption system, a pipeline of high-demand applications, and a regulatory fast track that could speed up approvals.
It’s a newly listed company, still flying under the radar, yet it’s targeting a $100B+ market and positioning itself in pain management, emergency treatment, and high-performance supplements.
The pieces are in motion, and with clinical trials, upcoming product launches, and strategic manufacturing partnerships, this is a profile that could start getting more attention sooner rather than later.
We will have all eyes on (ASBP) tomorrow morning.
Consider taking a look at (ASBP) before you call it a night.
Also, keep a lookout for my morning update—probably coming bright and early.
Have a great night. | 




ليست هناك تعليقات:
إرسال تعليق